Health Issues
Chinese Cresteds are Generally Healthy, but like all breeds, they can have certain health conditions. Not all Cresteds will get any or all of these diseases, but it's important to be aware of them.
You can expect to see health clearances from the Orthopedic Foundation for Animals (OFA) for hip and elbow dysplasia; from the Canine Eye Registry Foundation (CERF) certifying that eyes are normal; and from the Brainstem Auditory Evoked Response (BAER) which tests the dog's hearing status. Also tested is Patellar Luxation (PL) .
Dental Issues: This is due to a genetic link that exists between dominant hairlessness and missing teeth. The Hairless Crested has small teeth that can slope toward the front of the mouth and cause problems; the Powderpuff has normal toy breed dentition. The Hairless Cresteds often lose many teeth by the tender age of two or three. Some Hairless require canned food, while others eat kibble with no problem. See page Dentition
You can expect to see health clearances from the Orthopedic Foundation for Animals (OFA) for hip and elbow dysplasia; from the Canine Eye Registry Foundation (CERF) certifying that eyes are normal; and from the Brainstem Auditory Evoked Response (BAER) which tests the dog's hearing status. Also tested is Patellar Luxation (PL) .
Dental Issues: This is due to a genetic link that exists between dominant hairlessness and missing teeth. The Hairless Crested has small teeth that can slope toward the front of the mouth and cause problems; the Powderpuff has normal toy breed dentition. The Hairless Cresteds often lose many teeth by the tender age of two or three. Some Hairless require canned food, while others eat kibble with no problem. See page Dentition

Primary Lens Luxation (PLL) is a well-recognised, painful and blinding inherited eye condition that affects many breeds of dog, particularly terrier and terrier-type breeds including (but not restricted to) Miniature bull terriers, Tibetan terriers, Jack and Parson Russell terriers, Lancashire Heelers and Chinese Crested dogs, also the Australian Cattle Dog, Jagd Terrier, Patterdale Terrier, Rat Terrier, Sealyham Terrier, Tenterfield Terrier, Toy Fox Terrier, Volpino Italiano, Welsh Terrier, Wire-haired Fox Terrier and Yorkshire Terrier.
In affected dogs the zonular fibres which support the lens breakdown or disintegrate, causing the lens to fall into the wrong position within the eye. If the lens falls into the anterior chamber of the eye glaucoma and loss of vision can quickly result.
Scientists at the AHT have identified a mutation that is associated with the development of PLL in several breeds of dog. The DNA test we are now offering examines the DNA from each dog being tested for the presence or absence of this precise mutation. It is thus a ‘mutation-based test’ and not a ‘linkage-based test’
Breeders will be sent results identifying their dog as belonging to one of three categories:
CLEAR: these dogs have two normal copies of DNA. Our research has demonstrated clear dogs will not develop PLL as a result of the mutation we are testing for, although we cannot exclude the possibility they might develop PLL due to other causes, such as trauma or the effects of other, unidentified mutations.
CARRIER: these dogs have one copy of the mutation and one normal copy of DNA. Our research has demonstrated that carriers have a very low risk of developing PLL. The majority of carriers do not develop PLL during their lives but a small percentage do. We currently estimate that between 2% – 20% of carriers will develop the condition, although we believe the true percentage is nearer to 2% than 20%. We do not currently know why some carriers develop the condition whereas the majority do not, and we advise that all carriers have their eyes examined by a veterinary ophthalmologist every 6- 12 months, from the age of 2, throughout their entire lives.
GENETICALLY AFFECTED: these dogs have two copies of the mutation and will almost certainly develop PLL during their lifetime. We advise that all genetically affected dogs have their eyes examined by a veterinary ophthalmologist every 6 months, from the age of 18 months, so the clinical signs of PLL are detected as early as possible.
Breeding Advice
Our research has also demonstrated that the frequency of the PLL mutation is extremely high in the PLL-affected breeds that we have studied in depth. This means that allowing only CLEAR dogs to breed could have a devastating effect on breed diversity and substantially increase the likelihood of new inherited diseases emerging. Therefore, we strongly advise breeders to consider all their dogs for breeding, regardless of their PLL genotype. GENETICALLY AFFECTED and CARRIER dogs can be bred with, but should only be bred to DNA tested, CLEAR dogs. All puppies from any litter that has at least one CARRIER parent should be DNA tested, so that the CARRIERS can be identified and followed clinically throughout their lives. This practise should be followed for at least one or two generations, to allow the PLL mutation to be slowly eliminated from the population without severely reducing the genetic diversity of breeds at risk.
In affected dogs the zonular fibres which support the lens breakdown or disintegrate, causing the lens to fall into the wrong position within the eye. If the lens falls into the anterior chamber of the eye glaucoma and loss of vision can quickly result.
Scientists at the AHT have identified a mutation that is associated with the development of PLL in several breeds of dog. The DNA test we are now offering examines the DNA from each dog being tested for the presence or absence of this precise mutation. It is thus a ‘mutation-based test’ and not a ‘linkage-based test’
Breeders will be sent results identifying their dog as belonging to one of three categories:
CLEAR: these dogs have two normal copies of DNA. Our research has demonstrated clear dogs will not develop PLL as a result of the mutation we are testing for, although we cannot exclude the possibility they might develop PLL due to other causes, such as trauma or the effects of other, unidentified mutations.
CARRIER: these dogs have one copy of the mutation and one normal copy of DNA. Our research has demonstrated that carriers have a very low risk of developing PLL. The majority of carriers do not develop PLL during their lives but a small percentage do. We currently estimate that between 2% – 20% of carriers will develop the condition, although we believe the true percentage is nearer to 2% than 20%. We do not currently know why some carriers develop the condition whereas the majority do not, and we advise that all carriers have their eyes examined by a veterinary ophthalmologist every 6- 12 months, from the age of 2, throughout their entire lives.
GENETICALLY AFFECTED: these dogs have two copies of the mutation and will almost certainly develop PLL during their lifetime. We advise that all genetically affected dogs have their eyes examined by a veterinary ophthalmologist every 6 months, from the age of 18 months, so the clinical signs of PLL are detected as early as possible.
Breeding Advice
Our research has also demonstrated that the frequency of the PLL mutation is extremely high in the PLL-affected breeds that we have studied in depth. This means that allowing only CLEAR dogs to breed could have a devastating effect on breed diversity and substantially increase the likelihood of new inherited diseases emerging. Therefore, we strongly advise breeders to consider all their dogs for breeding, regardless of their PLL genotype. GENETICALLY AFFECTED and CARRIER dogs can be bred with, but should only be bred to DNA tested, CLEAR dogs. All puppies from any litter that has at least one CARRIER parent should be DNA tested, so that the CARRIERS can be identified and followed clinically throughout their lives. This practise should be followed for at least one or two generations, to allow the PLL mutation to be slowly eliminated from the population without severely reducing the genetic diversity of breeds at risk.
PRA DiseaseThe genetic disorder, prcd-PRA , causes cells in the retina at the back of the eye to degenerate and die, even though the cells seem to develop normally early in life. The “rod” cells operate in low light levels and are the first to lose normal function. Night blindness results. Then the “cone” cells gradually lose their normal function in full light situations. Most affected dogs will eventually be blind. Typically, the clinical disease is recognized first in early adolescence or early adulthood. Since age at onset of disease varies among breeds, you should read specific information for your dog. Diagnosis of retinal disease can be difficult. Conditions that seem to be prcd-PRA might instead be another disease and might not be inherited. OptiGen’s genetic test assists in making the diagnosis. It’s important to remember that not all retinal disease is PRA and not all PRA is the prcd form of PRA. Annual eye exams by a veterinary ophthalmologist will build a history of eye health that will help to diagnose disease.
Unfortunately, at this time there is no treatment or cure for PRA. If your dog is affected, you may find it helpful to read about other owners’ experiences living with blind dogs. (suggested links:www.eyevet.org and www.blinddogs.com)
InheritancePrcd-PRA is inherited as a recessive trait. This means a disease gene must be inherited from each parent in order to cause disease in an offspring. Parents were either “carrier” or affected. A carrier has one disease gene and one normal gene, and is termed “heterozygous” for the disease. A normal dog has no disease gene and is termed “homozygous normal” – both copies of the gene are the same. And a dog with two disease genes is termed “homozygous affected” – both copies of the gene are abnormal.
It’s been proven that all breeds being tested for prcd-PRA have the same disease caused by the same mutated gene. This is so, even though the disease might develop at different ages or with differing severity from one breed to another.
Although prcd-PRA is inherited, it can be avoided in future generations by testing dogs before breeding. Identification of dogs that do not carry disease genes is the key. These "clear" dogs can be bred to any mate - even to a prcd-affected dog which may be a desirable breeding prospect for other reasons. The chance of producing affected pups from such breedings depends on the certainty of test results. Again, you’ll find the specific information on certainty of test results for your dog by linking to breed specific information.
The Genetic TestThe OptiGen prcd test is done on a small sample of blood from the dog. The test analyzes the specific DNA mutation causing prcd-PRA. The OptiGen test detects the mutant, abnormal gene copy and the normal gene copy. The result of the test is a genotype and allows separation of dogs into three groups: Normal/Clear (homozygous normal), Carrier (heterozygous) and Affected (homozygous mutant).
Possible results using the OptiGen prcd test
Normal Normal/Clear - Can be bred to any dog, extremely low risk of producing affecteds , extremely low risk of prcd disease
Heterozygous Carrier - Should be bred only to Normal/Clear to remove risk of producing affecteds. Extremely low low risk of prcd disease
Homozygous Mutant - Affected. Should be bred only to Normal/Clear to remove risk of producing affecteds. Very high risk of prcd disease
Unfortunately, at this time there is no treatment or cure for PRA. If your dog is affected, you may find it helpful to read about other owners’ experiences living with blind dogs. (suggested links:www.eyevet.org and www.blinddogs.com)
InheritancePrcd-PRA is inherited as a recessive trait. This means a disease gene must be inherited from each parent in order to cause disease in an offspring. Parents were either “carrier” or affected. A carrier has one disease gene and one normal gene, and is termed “heterozygous” for the disease. A normal dog has no disease gene and is termed “homozygous normal” – both copies of the gene are the same. And a dog with two disease genes is termed “homozygous affected” – both copies of the gene are abnormal.
It’s been proven that all breeds being tested for prcd-PRA have the same disease caused by the same mutated gene. This is so, even though the disease might develop at different ages or with differing severity from one breed to another.
Although prcd-PRA is inherited, it can be avoided in future generations by testing dogs before breeding. Identification of dogs that do not carry disease genes is the key. These "clear" dogs can be bred to any mate - even to a prcd-affected dog which may be a desirable breeding prospect for other reasons. The chance of producing affected pups from such breedings depends on the certainty of test results. Again, you’ll find the specific information on certainty of test results for your dog by linking to breed specific information.
The Genetic TestThe OptiGen prcd test is done on a small sample of blood from the dog. The test analyzes the specific DNA mutation causing prcd-PRA. The OptiGen test detects the mutant, abnormal gene copy and the normal gene copy. The result of the test is a genotype and allows separation of dogs into three groups: Normal/Clear (homozygous normal), Carrier (heterozygous) and Affected (homozygous mutant).
Possible results using the OptiGen prcd test
Normal Normal/Clear - Can be bred to any dog, extremely low risk of producing affecteds , extremely low risk of prcd disease
Heterozygous Carrier - Should be bred only to Normal/Clear to remove risk of producing affecteds. Extremely low low risk of prcd disease
Homozygous Mutant - Affected. Should be bred only to Normal/Clear to remove risk of producing affecteds. Very high risk of prcd disease

Dry Eye (Keratoconjunctivitis Sicca) is a disorder of the tear glands that results in insufficient aqueous tear production and a correspondingly dry cornea. The tear film contains less of the aqueous layer and more of the mucus layer. In consequence, the classic sign of dry eye is a thick, stringy, mucoid to mucopurulent discharge.Since this type of discharge can also be seen with conjunctivitis, dogs with dry eye may be mistakenly treated for chronic conjunctivitis for long periods with little or no improvement. In a dog with dry eye, the bright, glistening sheen normally seen in the eye is replaced by a lackluster appearance in which the cornea is dry, dull, and opaque. Recurrent bouts of conjunctivitis are typical. Eventually the cornea becomes ulcerated or develops keratitis. Blindness may ensue.
Dry eye can have several causes. Immune-mediated diseases appear to play a major role. Other cases are idiopathic-that is, the cause is not known. Breeds predisposed to dry eye include Bulldogs, Cocker Spaniels, Lhasa Apsos, West Highland White Terriers, and others.
Some specific conditions that predispose a dog to dry eye include:
Treatment: For many years, the frequent application of artificial tears was the only treatment available for dry eye. But FDA approval of ophthalmic cyclosporin has revolutionized treatment and greatly improved results. Cyclosporin is an immunosuppressive drug that reverses immune-mediated destruction of the lacrimal glands.
Dry eye can have several causes. Immune-mediated diseases appear to play a major role. Other cases are idiopathic-that is, the cause is not known. Breeds predisposed to dry eye include Bulldogs, Cocker Spaniels, Lhasa Apsos, West Highland White Terriers, and others.
Some specific conditions that predispose a dog to dry eye include:
- Injury to the nerves that innervate the lacrimal glands. A branch of the facial nerve that activates the tear glands passes through the middle ear. Infections in the middle ear can damage this branch, affecting the tear glands as well as the muscles on that side of the face. In this case, the opposite eye is not affected.
- Injury to the tear glands themselves. Partial or complete destruction of tear glands can follow systemic diseases such as canine distemper, Addison’s disease, and immune-mediated diseases such as rheumatoid arthritis. Bacterial blepharitis or conjunctivitis can destroy the tear glands or occlude the small ducts that carry the tears into the eye. A number of sulfonamide drugs are toxic to tear glands. Tear gland injuries may be partially reversible if the underlying cause is eliminated.
- Congenital absence of the tear glands is rare, but may occur in the smaller breeds.
- Removal of the third eyelid or the lacrimal gland attached to it.
Treatment: For many years, the frequent application of artificial tears was the only treatment available for dry eye. But FDA approval of ophthalmic cyclosporin has revolutionized treatment and greatly improved results. Cyclosporin is an immunosuppressive drug that reverses immune-mediated destruction of the lacrimal glands.

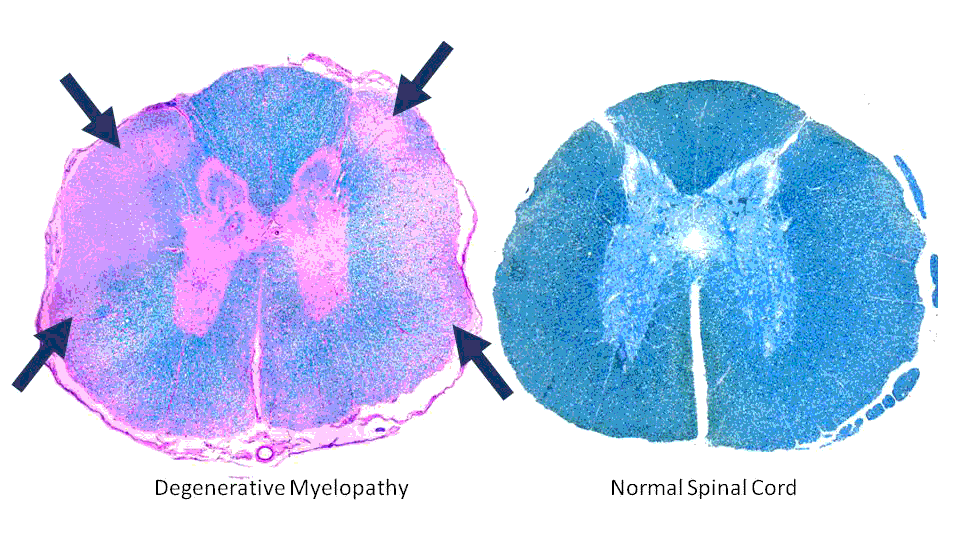
Degenerative myelopathy is a progressive disease of the spinal cord in older dogs. The disease has an insidious onset typically between 8 and 14 years of age. It begins with a loss of coordination (ataxia) in the hind limbs. The affected dog will wobble when walking, knuckle over or drag the feet. This can first occur in one hind limb and then affect the other. As the disease progresses, the limbs become weak and the dog begins to buckle and has difficulty standing. The weakness gets progressively worse until the dog is unable to walk. The clinical course can range from 6 months to 1 year before dogs become paraplegic. If signs progress for a longer period of time, loss of urinary and fecal continence may occur and eventually weakness will develop in the front limbs. Another key feature of DM is that it is not a painful disease.
Degenerative myelopathy begins with the spinal cord in the thoracic (chest) region. If we look under the microscope at that area of the cord from a dog that has died from DM, we see degeneration of the white matter of the spinal cord. The white matter contains fibers that transmit movement commands from the brain to the limbs and sensory information from the limbs to the brain.
This degeneration consists of both demyelination (stripping away the insulation of these fibers) and axonal loss (loss of the actual fibers), and interferes with the communication between the brain and limbs. Recent research has identified a mutation in a gene that confers a greatly increased risk of developing the disease.
How is degenerative myelopathy clinically diagnosed?
Degenerative myelopathy is a diagnosis of elimination. We look for other causes of the weakness using diagnostic tests like myelography and MRI. When we have ruled them out, we end up with a presumptive diagnosis of DM. The only way to confirm the diagnosis is to examine the spinal cord under the microscope when a necropsy (autopsy) is performed. There are degenerative changes in the spinal cord characteristic for DM and not typical for some other spinal cord disease.
What else can look like degenerative myelopathy?
Any disease that affects the dog’s spinal cord can cause similar signs of loss of coordination and weakness. Since many of these diseases can be treated effectively, it is important to pursue the necessary tests to be sure that the dog doesn’t have one of these diseases. The most common cause of hind limb weakness is herniated intervertebral disks. The disks are shock absorbers between the vertebrae in the back. When herniated, they can cause pressure on the spinal cord and weakness or paralysis. Short-legged, long back dogs are prone to slipped disks. A herniated disk can usually be detected with X-rays of the spine and myelogram or by using more advanced imaging such as CT scan or MRI. Other diseases we should consider include tumors, cysts, infections, injuries and stroke. Similar diagnostic procedures will help to diagnose most of these diseases. If necessary, your veterinarian can refer you to a board certified neurologist who can aid in diagnosing degenerative myelopathy. A directory to a neurologist near you can be found atAmerican College of Veterinary Internal Medicine website under the "Find a Specialist Near You" link.
How do we treat degenerative myelopathy?
There are no treatments that have been clearly shown to stop or slow progression of DM. Although there are a number of approaches that have been tried or recommended on the internet, no scientific evidence exists that they work. The outlook for a dog with DM is still grave. The discovery of a gene that identifies dogs at risk for developing degenerative myelopathy could pave the way for therapeutic trials to prevent the disease from developing. Meanwhile, the quality of life of an affected dog can be improved by measures such as good nursing care, physical rehabilitation, pressure sore prevention, monitoring for urinary infections, and ways to increase mobility through use of harnesses and carts.
EXPLANATION OF DM DNA TEST RESULTS
Normal (N/N)This dog is homozygous N/N for the mutation that is the most common cause of DM, with two normal copies of the gene. Among the hundreds of dogs studied so far at the University of Missouri, only two dogs with test results of N/N (Normal) have been confirmed to have DM. The N/N (Normal) dog can only transmit the normal counterpart of the common mutation to its offspring, and it is unlikely that this dog or its offspring will ever develop DM.
Carrier (A/N)This dog is heterozygous A/N, with one mutated copy of the gene and one normal copy of the gene, and is classified as a carrier. Carriers are far less likely to develop DM, but we have confirmed DM in a few carrier dogs. They may be used carefully in breeding programs to keep their good qualities while reducing risk of DM in future generations.
At-Risk (A/A)This dog is homozygous A/A, with two mutated copies of the gene, and is at risk for developing Degenerative Myelopathy (DM). Although almost all dogs in the research study with confirmed DM have had A/A DNA test results, recent evidence suggest that there are other causes of DM in some breeds. In addition, not all dogs testing as A/A have shown clinical signs of DM. DM is typically a late onset disease, and dogs testing as A/A that are clinically normal may still begin to show signs of the disease as they age. Some dogs testing A/A did not begin to show clinical signs of DM until they were 15 years of age. Research is ongoing to estimate what percentage of dogs testing as A/A will develop DM within their lifespan. At this point, the mutation can only be interpreted as being at risk of developing DM within the animal's life. For dogs showing clinical signs with a presumptive diagnosis of DM, affected (A/A) test results can be used as an additional tool to aid in the diagnosis of DM. Dogs testing At-Risk (A/A) can only pass the mutated gene on to their offspring.
EquivocalAn Equivocal test result indicates that the test results were inconclusive. This is typically the result of poor sample collection. When the test yields an equivocal result, a second punch will be taken from the FTA card and the test rerun. If the second test is still equivocal, the owner will be contacted and asked to submit a new sample.
Degenerative myelopathy begins with the spinal cord in the thoracic (chest) region. If we look under the microscope at that area of the cord from a dog that has died from DM, we see degeneration of the white matter of the spinal cord. The white matter contains fibers that transmit movement commands from the brain to the limbs and sensory information from the limbs to the brain.
This degeneration consists of both demyelination (stripping away the insulation of these fibers) and axonal loss (loss of the actual fibers), and interferes with the communication between the brain and limbs. Recent research has identified a mutation in a gene that confers a greatly increased risk of developing the disease.
How is degenerative myelopathy clinically diagnosed?
Degenerative myelopathy is a diagnosis of elimination. We look for other causes of the weakness using diagnostic tests like myelography and MRI. When we have ruled them out, we end up with a presumptive diagnosis of DM. The only way to confirm the diagnosis is to examine the spinal cord under the microscope when a necropsy (autopsy) is performed. There are degenerative changes in the spinal cord characteristic for DM and not typical for some other spinal cord disease.
What else can look like degenerative myelopathy?
Any disease that affects the dog’s spinal cord can cause similar signs of loss of coordination and weakness. Since many of these diseases can be treated effectively, it is important to pursue the necessary tests to be sure that the dog doesn’t have one of these diseases. The most common cause of hind limb weakness is herniated intervertebral disks. The disks are shock absorbers between the vertebrae in the back. When herniated, they can cause pressure on the spinal cord and weakness or paralysis. Short-legged, long back dogs are prone to slipped disks. A herniated disk can usually be detected with X-rays of the spine and myelogram or by using more advanced imaging such as CT scan or MRI. Other diseases we should consider include tumors, cysts, infections, injuries and stroke. Similar diagnostic procedures will help to diagnose most of these diseases. If necessary, your veterinarian can refer you to a board certified neurologist who can aid in diagnosing degenerative myelopathy. A directory to a neurologist near you can be found atAmerican College of Veterinary Internal Medicine website under the "Find a Specialist Near You" link.
How do we treat degenerative myelopathy?
There are no treatments that have been clearly shown to stop or slow progression of DM. Although there are a number of approaches that have been tried or recommended on the internet, no scientific evidence exists that they work. The outlook for a dog with DM is still grave. The discovery of a gene that identifies dogs at risk for developing degenerative myelopathy could pave the way for therapeutic trials to prevent the disease from developing. Meanwhile, the quality of life of an affected dog can be improved by measures such as good nursing care, physical rehabilitation, pressure sore prevention, monitoring for urinary infections, and ways to increase mobility through use of harnesses and carts.
EXPLANATION OF DM DNA TEST RESULTS
Normal (N/N)This dog is homozygous N/N for the mutation that is the most common cause of DM, with two normal copies of the gene. Among the hundreds of dogs studied so far at the University of Missouri, only two dogs with test results of N/N (Normal) have been confirmed to have DM. The N/N (Normal) dog can only transmit the normal counterpart of the common mutation to its offspring, and it is unlikely that this dog or its offspring will ever develop DM.
Carrier (A/N)This dog is heterozygous A/N, with one mutated copy of the gene and one normal copy of the gene, and is classified as a carrier. Carriers are far less likely to develop DM, but we have confirmed DM in a few carrier dogs. They may be used carefully in breeding programs to keep their good qualities while reducing risk of DM in future generations.
At-Risk (A/A)This dog is homozygous A/A, with two mutated copies of the gene, and is at risk for developing Degenerative Myelopathy (DM). Although almost all dogs in the research study with confirmed DM have had A/A DNA test results, recent evidence suggest that there are other causes of DM in some breeds. In addition, not all dogs testing as A/A have shown clinical signs of DM. DM is typically a late onset disease, and dogs testing as A/A that are clinically normal may still begin to show signs of the disease as they age. Some dogs testing A/A did not begin to show clinical signs of DM until they were 15 years of age. Research is ongoing to estimate what percentage of dogs testing as A/A will develop DM within their lifespan. At this point, the mutation can only be interpreted as being at risk of developing DM within the animal's life. For dogs showing clinical signs with a presumptive diagnosis of DM, affected (A/A) test results can be used as an additional tool to aid in the diagnosis of DM. Dogs testing At-Risk (A/A) can only pass the mutated gene on to their offspring.
EquivocalAn Equivocal test result indicates that the test results were inconclusive. This is typically the result of poor sample collection. When the test yields an equivocal result, a second punch will be taken from the FTA card and the test rerun. If the second test is still equivocal, the owner will be contacted and asked to submit a new sample.

Legg-Calve-Perthes Disease (LCP) is a disorder of hip joint conformation occurring in both humans and dogs. In dogs, it is most often seen in the miniature and toy breeds between the ages of 4 months to a year.
LCP results when the blood supply to the femoral head is interrupted resulting in avascular necrosis, or the death of the bone cells. Followed by a period of revascularization, the femoral head is subject to remodeling and/or collapse creating an irregular fit in the acetabulum, or socket. This process of bone cells dying and fracturing followed by new bone growth and remodeling of the femoral head and neck, can lead to stiffness and pain.
LCP is believed to be an inherited disease, although the mode of inheritance is not known. Because there is a genetic component, it is recommended that dogs affected with LCP not be used in breeding programs.
In an effort to assist breeders in establishing a control program to limit the prevalence of the LCP, the OFA offers a health database specific to LCP. The OFA evaluations and the subsequent database of information will allow breeders to make more informed breeding decisions.
To receive an OFA LCP number based on a previous hip evaluation, owners should complete the appropriate application and the OFA will assign an LCP number. Evaluation fees will be refunded for dogs determined by the OFA to be affected.
LCP results when the blood supply to the femoral head is interrupted resulting in avascular necrosis, or the death of the bone cells. Followed by a period of revascularization, the femoral head is subject to remodeling and/or collapse creating an irregular fit in the acetabulum, or socket. This process of bone cells dying and fracturing followed by new bone growth and remodeling of the femoral head and neck, can lead to stiffness and pain.
LCP is believed to be an inherited disease, although the mode of inheritance is not known. Because there is a genetic component, it is recommended that dogs affected with LCP not be used in breeding programs.
In an effort to assist breeders in establishing a control program to limit the prevalence of the LCP, the OFA offers a health database specific to LCP. The OFA evaluations and the subsequent database of information will allow breeders to make more informed breeding decisions.
- Owners submit radiographs of their dogs in the standard hip extended ventrodorsal view endorsed by the AVMA.
- Dogs must be a minimum of 12 months of age on the date of the radiograph to be eligible for an LCP number.
- The radiographs may be taken by any veterinarian, but must contain the required dog identification in the film emulsion, exhibit proper positioning, and must be of sufficient quality for the OFA to reach a diagnosis.
- The radiographs along with the completed application and $25 evaluation fee are submitted to the OFA for review.
- At the time of submission, the owner selects whether any abnormal finding may be released in the public domain. All normal results are in the public domain and are available on the OFA website.
- A Board Certified Radiologist reviews the radiograph for evidence of Legg-Calve-Perthes.
- Phenotypically normal dogs are assigned an OFA Legg-Calve-Perthes number.
- Dogs with evidence of LCP are not assigned a number, but the OFA will issue a report stating the findings.
- The OFA submits quarterly reports to the parent club containing the dogs receiving LCP numbers, as well as any overall aggregate statistical data.
To receive an OFA LCP number based on a previous hip evaluation, owners should complete the appropriate application and the OFA will assign an LCP number. Evaluation fees will be refunded for dogs determined by the OFA to be affected.

CARDIAC GUIDELINES FOR BREEDERS
A careful clinical examination that emphasizes cardiac auscultation is the most expedient and cost-effective method for identifying Congenital Heart Disease in dogs. While there are exceptions, virtually all common congenital heart defects are associated with the presence of a cardiac murmur. Consequently, it is recommended that cardiac auscultation be the primary screening method for initial identification of CHD and the initial classification of dogs. Murmurs related to CHD may at times be difficult to distinguish from normal, innocent (also called physiologic or functional) murmurs. Innocent cardiac murmurs are believed to the related to normal blood flow in the circulation. Innocent murmurs are most common in young, growing animals. The prevalence of innocent heart murmurs in mature dogs (especially in athletic dogs) is undetermined. A common clinical problem is the distinction between innocent murmurs and murmurs arising from CHD.
Definitive diagnosis of CHD usually involves one or more of the following methods:
A careful clinical examination that emphasizes cardiac auscultation is the most expedient and cost-effective method for identifying Congenital Heart Disease in dogs. While there are exceptions, virtually all common congenital heart defects are associated with the presence of a cardiac murmur. Consequently, it is recommended that cardiac auscultation be the primary screening method for initial identification of CHD and the initial classification of dogs. Murmurs related to CHD may at times be difficult to distinguish from normal, innocent (also called physiologic or functional) murmurs. Innocent cardiac murmurs are believed to the related to normal blood flow in the circulation. Innocent murmurs are most common in young, growing animals. The prevalence of innocent heart murmurs in mature dogs (especially in athletic dogs) is undetermined. A common clinical problem is the distinction between innocent murmurs and murmurs arising from CHD.
Definitive diagnosis of CHD usually involves one or more of the following methods:
- Echocardiography with Doppler studies
- Cardiac catheterization with angiocardiography
- Post-mortem examination of the heart (necropsy).
- The noninvasive method of echocardiography with Doppler is the preferred method for establishing a definitive diagnosis in dogs when CHD is suspected the clinical examination. Echocardiography is an inappropriate screening tool for the identification of congenital heart disease and should be performed only when the results of clinical examinations suggest a definite or potential cardiovascular abnormality.
- Two-dimensional echocardiography provides an anatomic image of the heart and blood vessels. While moderate to severe cardiovascular malformations can generally be recognized by two-dimensional echocardiography, mild defects (which are often of great concern to breeders of dogs) may not be identifiable by this method alone.
- Doppler studies, including pulsed-wave and continuous wave spectral Doppler, and two-dimensional color Doppler demonstrate the direction and velocity of blood flow in the heart and blood vessels. Abnormal patterns of blood flow are best recognized by Doppler studies. Results of Doppler studies can be combined with those of the two-dimensional echocardiogram in assessing the severity of CHD. Color Doppler echocardiography is used to evaluate relatively large areas of blood flow and is beneficial in the overall assessment of the dog with suspected CHD. Turbulence maps employed in color Doppler imaging are useful for identifying high velocity or disturbed blood flow but are not sufficiently specific (or uniform among manufacturers) to quantify blood velocity. It is emphasized that quantitation of suspected blood flow abnormalities is essential and can only be accomplished with pulsed or continuous wave Doppler studies. Pulsed wave and continuous wave Doppler examinations provide a display of blood velocity spectra in a graphical format and are the methods of choice for assessing blood flow patterns and blood flow velocity in discrete anatomic areas.
- Cardiac catheterization is an invasive method for identification of CHD that is considered very reliable for the diagnosis of CHD. Cardiac catheterization should be performed by a cardiologist, usually requires general anesthesia, carries a small but definite procedural risk, and is generally more costly than noninvasive studies. While cardiac catheterization with angiocardiography is considered one of the standards for the diagnosis CHD, this method has been supplanted by echocardiography with Doppler for routine evaluation of suspected CHD.
- Necropsy examination of the heart should be done in any breeding dog that dies or is euthanized The hearts of puppies and dogs known to have cardiac murmurs should always be examined following the death of the animal. A post mortem examination of the heart is best done by a cardiologist or pathologist with experience in evaluating CHD. While it is obvious that necropsy cannot be used as a screening method, the information provided by this examination can be useful in guiding breeders and in establishing the modes of inheritance of CHD.
- The results to the examinations described above are most reliable when performed by an experienced individual with advanced training an experience in cardiovascular diagnosis. Echocardiography with Doppler, cardiac catheterization, and post-mortem examination of the heart for CHD requires advanced training in cardiovascular diagnostic methods and the pathology and pathophysiology of CHD.
- Examinations performed in mature dogs are most likely to be definitive. This is especially true when considering mild congenital heart defects. Innocent heart murmurs are less common in mature animals than in puppies are less likely to be a source of confusion. Furthermore, the murmurs associated with some mild congenital malformations become more obvious after a dog has reached maturity. While it is quite reasonable to perform preliminary evaluations and provide provisional certification to puppies and young dogs between 8 weeks and 1 year of age, final certification, prior to breeding, should be obtained in mature dogs at 12 months of age or older.
- Examination conditions must be appropriate for recognition of subtle cardiac malformations. Identification of soft cardiac murmurs is impeded by extraneous noise or by poorly restrained, anxious, or panting dogs.
- A standardized cardiac clinical examination must be performed according to a predetermined and clearly communicated protocol. Physical examination and cardiac auscultation should be used as the initial method of cardiac evaluation. If the clinical (as indicated above).
- Examiners who perform echocardiography with Doppler must use appropriate ultrasound equipment, transducers, and techniques. Such individuals should have advanced training in noninvasive cardiac diagnosis and should follow diagnostic standards established by their hospital and by the veterinary scientific community, including standards published by the American College of Veterinary Internal Medicine, specialty of Cardiology (J Vet Internal Med 1993;7:247-252).